Modeling neuro-glio-vascular interactions
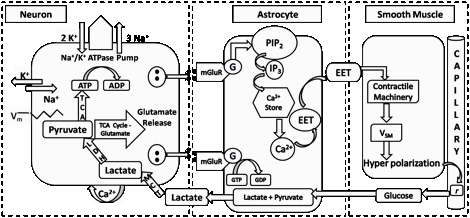
We present a model of neuron-astrocyte-vessel interacting in bidirectional fashion. Most models of cerebral circulation describe neurovascular interactions exclusively in the “forward” neuronàastrocyteàvessel direction. The proposed model indicates possibility of “reverse” influence also, with vasomotion rhythms influencing neural firing patterns. The model suggests that brain’s computations may be more comprehensively understood in terms of neuro-glial-vascular dynamics and not in terms of neural firing alone.
- Bankim Chander and V.S.Chakravarthy, A computational model of neuro-glio-vascular loop interactions, (under review).
- Bankim Chander, Srinivasa Chakravarthy, A Biophysical Model of Neuro‐Glial‐Vascular Interactions, 3rd Interlational conference on cognitive neurodynamics, Hilton Niseko village, Hokkaido, Japan, June 9-13,2011.
- Bankim Chander and V.S.Chakravarthy, A computational model of neuro-glio-vascular loop interactions, (under review).
We had earlier presented a network model of neuro-glio-vascular interactions. The model is an answer to the following question: why is there is a large glial layer mediating the interactions between neurons and microvessels?
- Rohit Gandrakota, V. S. Chakravarthy, Ranjan K. Pradhan, A model of indispensability of a large glial layer in cerebrovascular circulation. Neural Computation 2010 Apr;22(4):949-68.
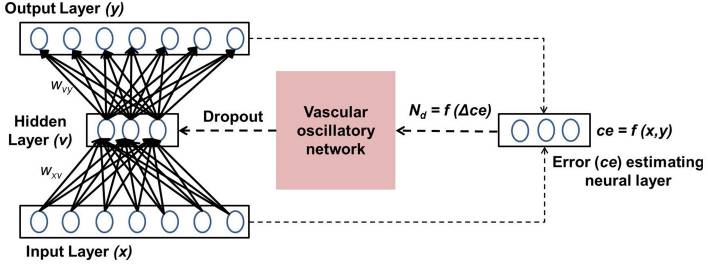
Cerebral vascular dynamics are generally thought to be controlled by neural activity in a unidirectional fashion. However, both computational modeling and experimental evidence point to the feedback effects of vascular dynamics on neural activity. Vascular feedback in the form of glucose and oxygen controls neuronal ATP, either directly or via the agency of astrocytes, which in turn modulates neural firing. Recently, a detailed model of the neuron-astrocyte-vessel system has shown how vasomotion can modulate neural firing. Similarly, arguing from known cerebrovascular physiology, an approach known as "hemoneural hypothesis" postulates functional modulation of neural activity by vascular feedback. To instantiate this perspective, we present a computational model in which a network of "vascular units" supplies energy to a neural network. The complex dynamics of the vascular network, modeled by a network of oscillators, turns neurons ON and OFF randomly. The informational consequence of such dynamics is explored in the context of an auto-encoder network. In the proposed model, each vascular unit supplies energy to a subset of hidden neurons of an autoencoder network, which constitutes its "projective field." Neurons that receive adequate energy in a given trial have reduced threshold, and thus are prone to fire. Dynamics of the vascular network are governed by changes in the reconstruction error of the auto-encoder network, interpreted as the neuronal demand. Vascular feedback causes random inactivation of a subset of hidden neurons in every trial. We observe that, under conditions of desynchronized vascular dynamics, the output reconstruction error is low and the feature vectors learnt are sparse and independent. Our earlier modeling study highlighted the link between desynchronized vascular dynamics and efficient energy delivery in skeletal muscle. We now show that desynchronized vascular dynamics leads to efficient training in an auto-encoder neural network
Philips RT, Chhabria K, Chakravarthy VS. Vascular Dynamics Aid a Coupled Neurovascular Network Learn Sparse Independent Features: A Computational Model. Frontiers in Neural Circuits. 2016;10:7. doi:10.3389/fncir.2016.00007
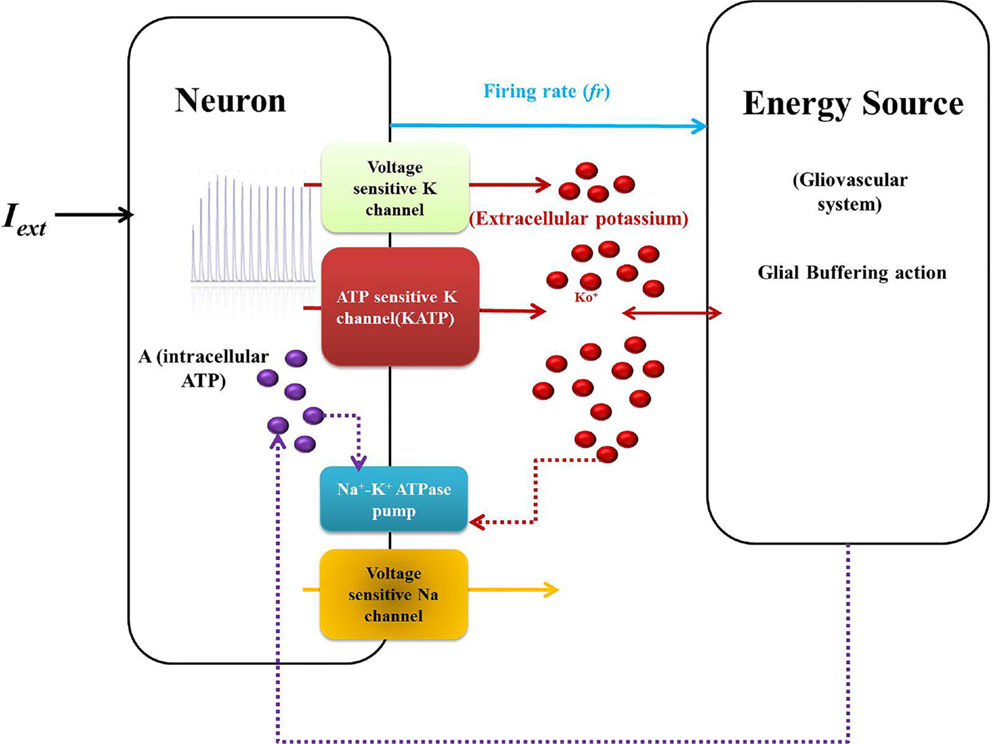
The motivation of developing simple minimal models for neuro-glio-vascular (NGV) system arises from a recent modeling study elucidating the bidirectional information flow within the NGV system having 89 dynamic equations (1). While this was one of the first attempts at formulating a comprehensive model for neuro-glio-vascular system, it poses severe restrictions in scaling up to network levels. On the contrary, low-dimensional models are convenient devices in simulating large networks that also provide an intuitive understanding of the complex interactions occurring within the NGV system. The key idea underlying the proposed models is to describe the glio-vascular system as a lumped system, which takes neural firing rate as input and returns an "energy" variable (analogous to ATP) as output. To this end, we present two models: biophysical neuro-energy (Model 1 with five variables), comprising KATP channel activity governed by neuronal ATP dynamics, and the dynamic threshold (Model 2 with three variables), depicting the dependence of neural firing threshold on the ATP dynamics. Both the models show different firing regimes, such as continuous spiking, phasic, and tonic bursting depending on the ATP production coefficient, ɛp, and external current. We then demonstrate that in a network comprising such energy-dependent neuron units, ɛp could modulate the local field potential (LFP) frequency and amplitude. Interestingly, low-frequency LFP dominates under low ɛp conditions, which is thought to be reminiscent of seizure-like activity observed in epilepsy. The proposed "neuron-energy" unit may be implemented in building models of NGV networks to simulate data obtained from multimodal neuroimaging systems, such as functional near infrared spectroscopy coupled to electroencephalogram and functional magnetic resonance imaging coupled to electroencephalogram. Such models could also provide a theoretical basis for devising optimal neurorehabilitation strategies, such as non-invasive brain stimulation for stroke patients.
Chhabria K,Chakravarthy VS, Low-dimensional models of 'Neuro-glio-vascular unit' for describing neural dynamics under normal and energy-starved conditions, Frontiers in Neurology,2016.