
Bioprocess development for xylitol, arabitol production by halotolerant yeast Debaryomyces nepalensis
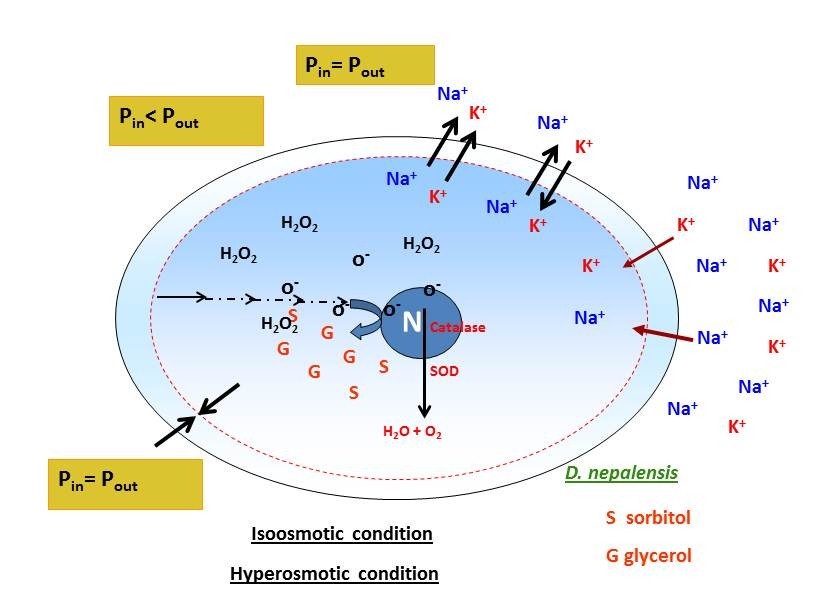
This strain was isolated from rotten apple from Velachery market, Chennai which is capable of growing using pectin as the sole source of carbon. Very few strains are known to produce both pectic lyases (pectinlyase and pectatelyase) simultaneously and their levels are low. This strain was known to produce both the enzymes at appreciable levels. Medium development using natural carbon source (lemon peel) was developed for production of both pectic lyases and optimized the physiological conditions in bioreactor. Under optimal conditions, the strain produced 2300 U/L. 26S DNA analysis revealed that this strain belong to Debaryomyces nepalensis. It has been demonstrated that D. nepalensis is a halotolerant strain and the physiological basis for the tolerance were determined. Based on our result, we hypothesized that there exists a balanced efflux and synthesis of osmolytes when D. nepalensis was exposed to hypoosmotic and hyperosmotic stress condition respectively. Our findings suggest that D. nepalensis is Na+ excluder yeast and it has an efficient transport system for sodium extrusion.
The kinetics of growth and polyol production by Debaryomyces nepalensis NCYC 3413 was studied under single and mixed substrate conditions. In the presence of glucose, the strain produced ethanol (35.8 ± 2.3 g/l), glycerol (9.0 ± 0.2 g/l) and arabitol (6.3 ± 0.2 g/l). In the presence of xylose, the strain produced xylitol (38 ± 1.8 g/l) and glycerol (18 ± 1.0 g/l) as major metabolites. important enzymes involved in the synthesis of xylitol (XR, xylose reductase and XDH, xylitol dehydrogenase), glycerol (G3PDH, glycerol-3-phosphate dehydrogenase) and ethanol (ADH, alcohol dehydrogenase) in cells grown in the presence of glucose and xylose revealed high specific activity of G3PDH and ADH in cells grown in the presence of glucose, whereas high specific activity of XR, XDH and G3PDH was observed in cells grown in the presence of xylose.
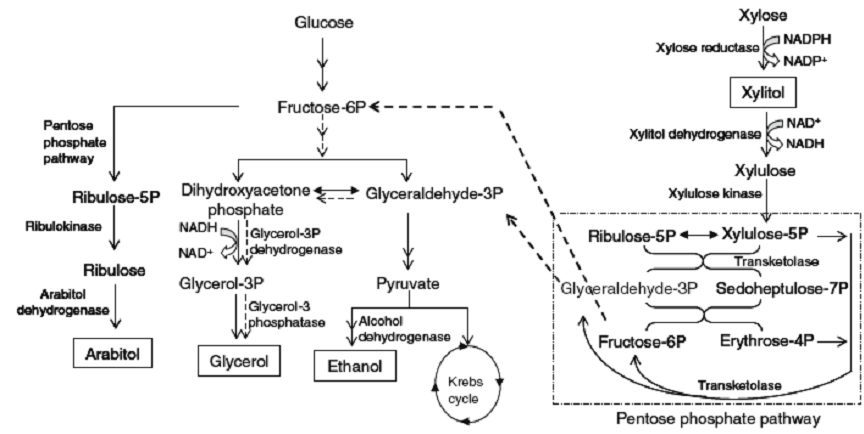
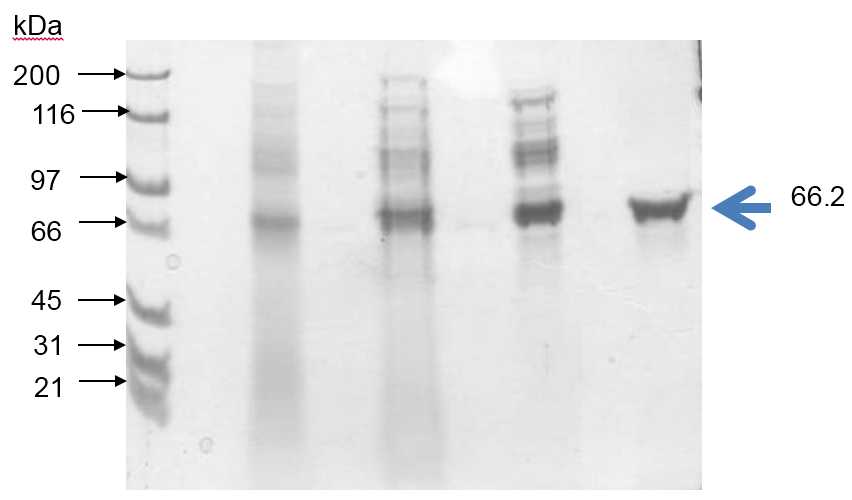
Xylose reductase (XR) from halotolerant yeast, Debaryomyces nepalensis NCYC 3413 was purified to apparent homogeneity. The enzyme has a molecular mass of 74 kDa with monomeric subunit of 36.4 kDa (MALDI-TOF/MS) and pI of 6.0. The enzyme exhibited its maximum activity at pH 7.0 and 45 oC (21.2 U/mg).
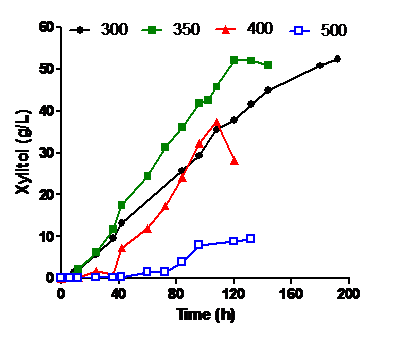
The enzyme exhibited high preference for pentoses over hexoses with greater catalytic efficiency for arabinose than xylose. The enzyme also showed absolute specificity with NADPH over NADH. The enzyme retained 90% activity with 100 mM of NaCl or KCl and 40% activity with 1 M KCl which suggest that the enzyme is moderately halotolerant. The medium constituents were screened and optimized for xylitol production from xylose. Currently cloning of XR and process optimization for xylitol and arabitol production in bioreactors is under progress.
