
Human phospholipid scramblases (hPLSCRs)
Phospholipid scramblases are a group of homologous proteins that are conserved in all eukaryotic organisms. They are believed to be involved in destroying plasma membrane phospholipid asymmetry at critical cellular events like cell activation, injury and apoptosis. However, a detailed mechanism of phospholipid scrambling still awaits a proper understanding. The most studied member of this family, phospholipid scramblase 1 (PLSCR1) (a 37 kDa protein), is involved in rapid Ca2+ dependent transbilayer redistribution of plasma membrane phospholipids. Recently the function of PLSCR1 is being challenged and evidences are giving strength to PLSCR1 acting as a signaling molecule. Our main objective is to determine the exact role of these domains in terms of calcium binding and PL scrambling and understanding the mechanism of PL flip-flop.
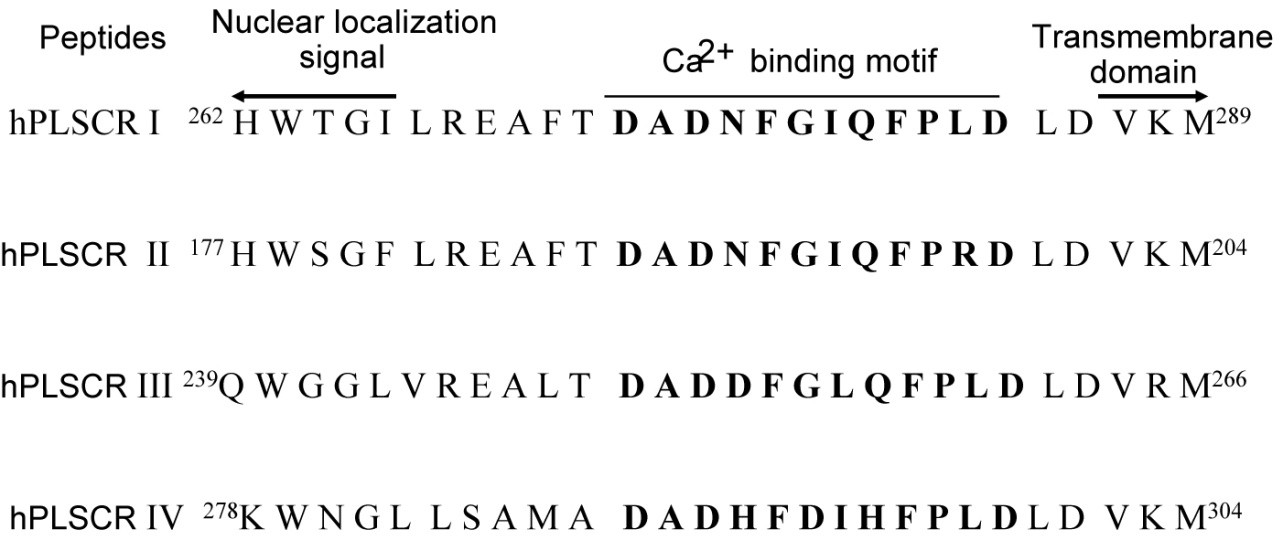
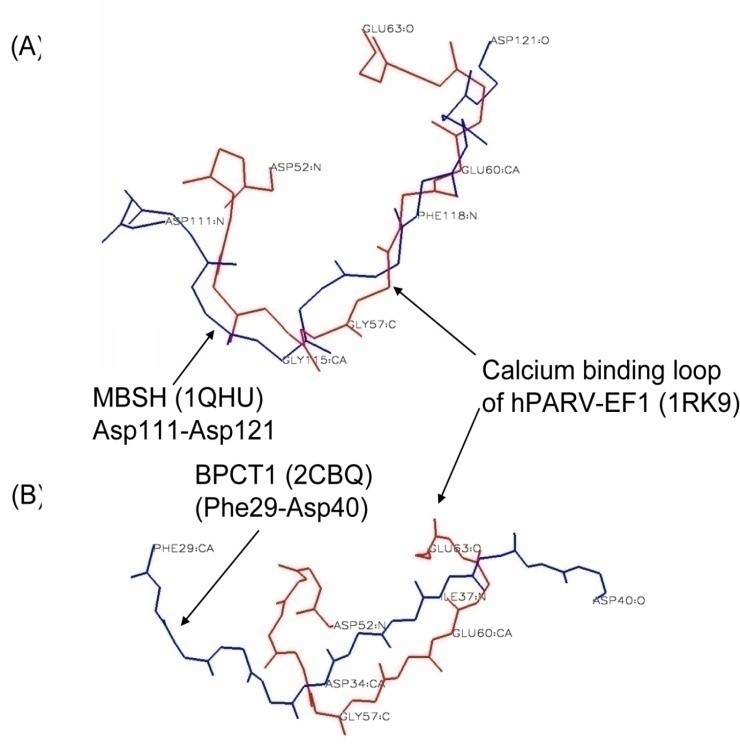
We analyzed the Ca2+ and Mg2+ binding to the calcium binding motifs of hPLSCR1-4 and hPLSCR1 by spectroscopic methods and isothermal titration calorimetry. The results in this study show that (i) affinities of the peptides are in the order hPLSCR1>hPLSCR3>hPLSCR2>hPLSCR4 for Ca2+ and in the order hPLSCR1>hPLSCR2>hPLSCR3>hPLSCR4 for Mg2+ and the affinity of Ca2+ and Mg2+ binding to protein hPLSCR1 was similar to that of the peptide I. Based on our experimental and theoretical work, we proposed that Ca2+ binding motifs present in scramblase family members represent a new class of low affinity Ca2+ binding motifs and scramblase like Ca2+ binding motifs are also present in other unrelated proteins that are known to exhibit changes in biochemical parameters upon binding to Ca2+ and other divalent metal ions.
In order to characterize the protein, we also developed expression and purification protocols. Over-expression of recombinant hPLSCR1 in E. coli BL21 (DE3) leads to its deposition in inclusion bodies (IBs). N-Lauroyl sarcosine was used to solubilise IBs and to recover functionally active hPLSCR1 from them. Protein was purified to homogeneity by Ni-NTA affinity chromatography and was >98% pure.
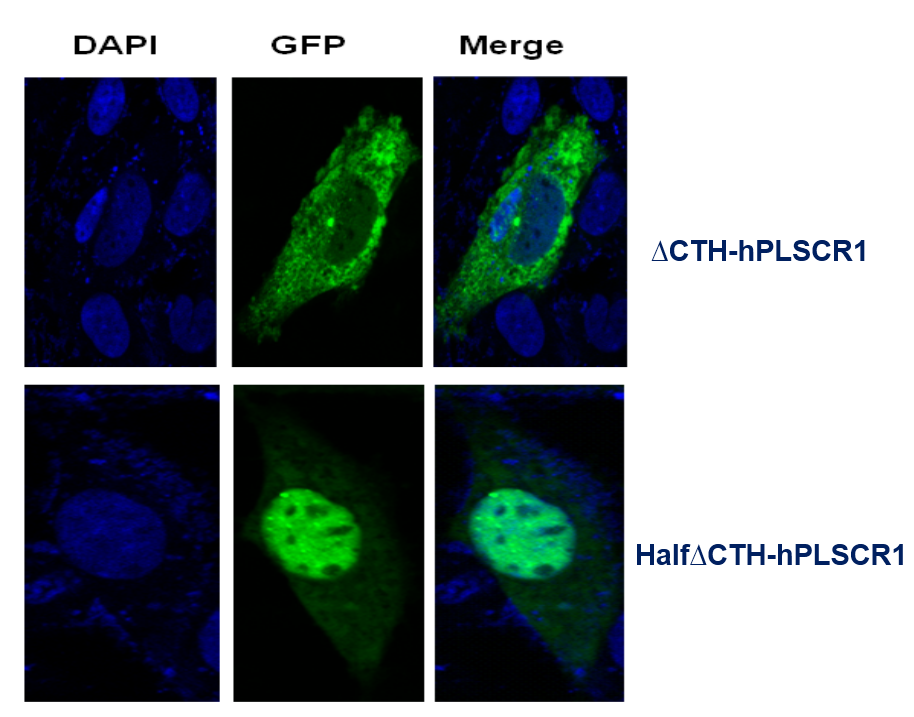
C-terminal helix (CTH) domain of hPLSCR1 was believed to be putative single transmembrane helix at the C-terminus. By deletion studies, we showed that CTH deletion lead to loss of activity of the protein and reduction in calcium binding suggesting that CTH is required for membrane insertion, Ca2+ co-ordination and also plays an important role in the functional conformation of hPLSCR1.
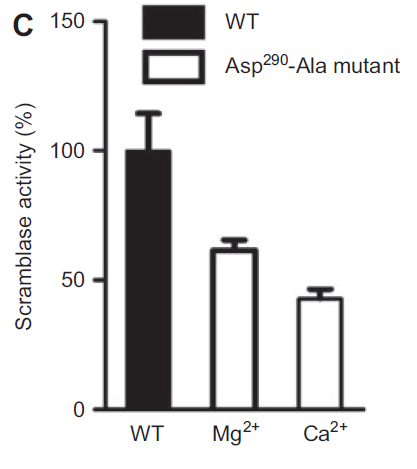
Human phospholipid scramblase 4 (hPLSCR4), an isoform of the scramblase family is a type II single pass transmembrane protein whose function remains unknown. To understand its role, recombinant hPLSCR4 was obtained by cloning the ORF into pET28 a(+) vector and overexpressed in E. coli. Functional assay showed that Ca2+, Mg2+ and Zn2+ activate hPLSCR4 and mediates scrambling activity independent of the phospholipid head group. Point mutation (Asp290 Ala) in hPLSCR4 decreased the Ca2+ binding affinity as well as Tb3+ luminescence, suggesting residues of the predicted Ca2+ binding motif are involved in Ca2+ binding. Functional reconstitution with (Asp290 Ala) mutant lead to ~50% and ~40% decrease in scramblase activity in presence of Ca2+ and Mg2+ respectively.
hPLSCR1 was also reported to be overexpressed during heavy metal poisoning. To check this, we assayed recombinant hPLSCR1 with Hg2+ and Pb2+.
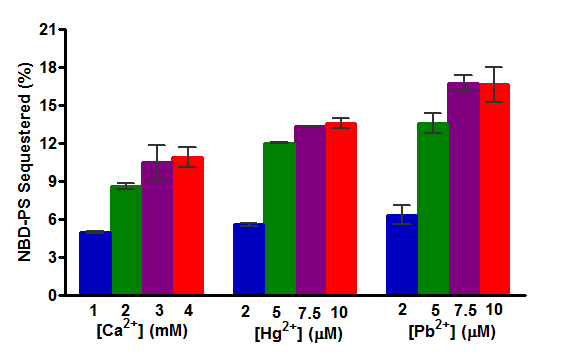
Activity was strongly induced by the addition of heavy metals when compared to calcium and this could say that hPLSCR1 can play a role in heavy metal poisoning.
Currently, the role of hPLSCR1 in apoptosis, cell development and in other disease pathways are under progress.
s
