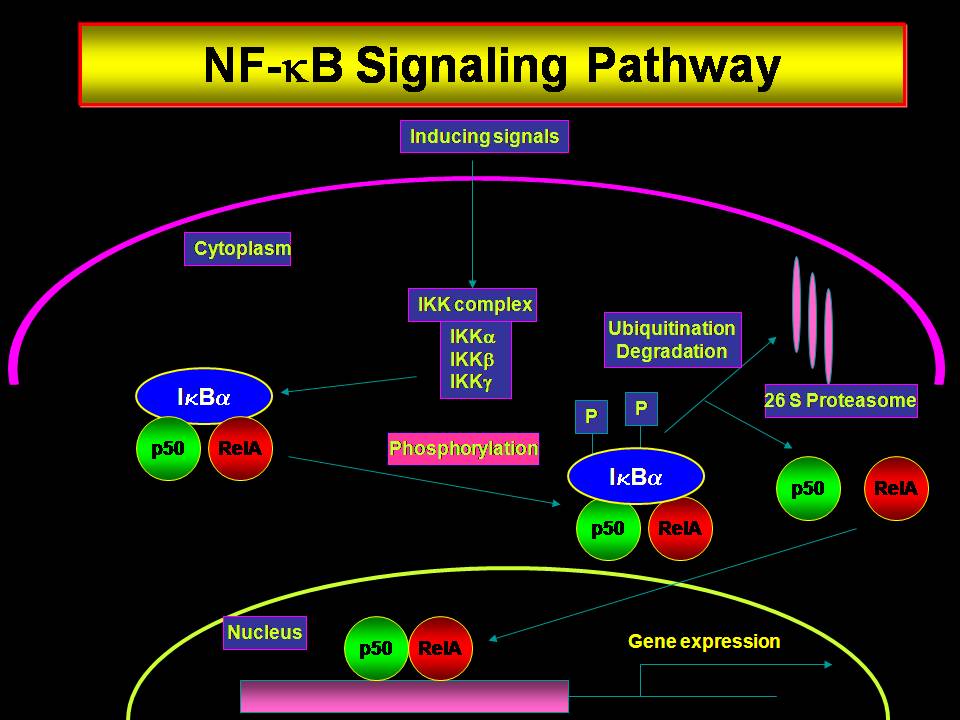
The ubiquitous transcription factor, NF-κB, is deregulated in many solid and hematological malignancies. Most of the tumor types and cell lines show constitutive activation of NF-κB that enables them to resist chemo and/or radio therapy by inducing the expression of anti apoptotic/survival factors. In addition NF-κB enhances the ability of tumors to metastasize as well as form new vasculature, which are critical for tumor survival. Since constitutive NF-κB activity correlates positively to the resistance of cancer cells to anti cancer agents, antagonizing NF-κB offers an immense therapeutic potential to improve the clinical response in these tumor types. Many studies have shown that downregulation of NF-κB sensitizes cells to therapy as well as inhibits the metastatic and angiogenic ability of different tumor types. Recently we have reported that NF-κB is constitutively activated in high grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. However, the mechanisms by which tumors maintain a sustained activation of NF-κB are not clearly defined. Since ex vivo tissue explants are not very much suited for mechanistic studies, the present proposal is aimed at identifying the factors responsible for the activation of NF-κB and their targets in human cervical cancers using in vitro model systems. Hence it is proposed to study the expression of activators of NF-κB and their targets which are often responsible for tumor initiation, progression, invasion and metastasis. It is proposed to test the hypothesis that cytokines and growth factors secreted by tumor cells as well as the surrounding stromal layer are responsible for constitutive activation of NF-κB. Organotypic raft culture will be used as a mechanistic model to test this hypothesis and it will also serve as an in vitro model for testing the efficacy of anti cancer drugs.